Fortunately, optometric practice rarely requires exposure-prone or invasive procedures, which are highly associated with an increased risk of transmitting blood-borne infections between patient and healthcare worker.
There are, however, other risks of cross-transmission of infection that the practice of optometry presents, with the greatest risk being eye infections that may be bacterial, viral, chlamydial, fungal or acanthamoeba. Adherence to strict and appropriate hygiene protocols may significantly reduce and even eliminate the risk of possible cross-transmission in healthcare workers and patients.
RISK OF CROSS-TRANSMISSION IN OPTOMETRY
Various risks of infection need to be considered in optometry. There are two main areas that need to be addressed in practice, namely direct person to person transmission, and transmission via a contaminated object or surface (1).
DIRECT TRANSMISSION:
In practice, direct transmission of infection occurs via four main routes:

Physical contact between a physician/technician and a patient who presents with:
• An ophthalmic bacterial or viral infection, such as conjunctivitis
• A skin infection such as staphylococcus, herpes or fungi, for example
• An enteric infection, such as gastroenteritis

Through airborne contraction as a result of a respiratory illness such as influence, tuberculosis or coronavirus. Airborne infections present a risk to both physician/technician and patient due to the close proximity of the work near the nose and mouth. Aerosols that are generated when a person coughs, sneezes or speaks loudly also pose a risk of transmission due to their nature to remain airborne for short periods of time if they’re larger particles, or longer periods of time if they’re smaller particles.
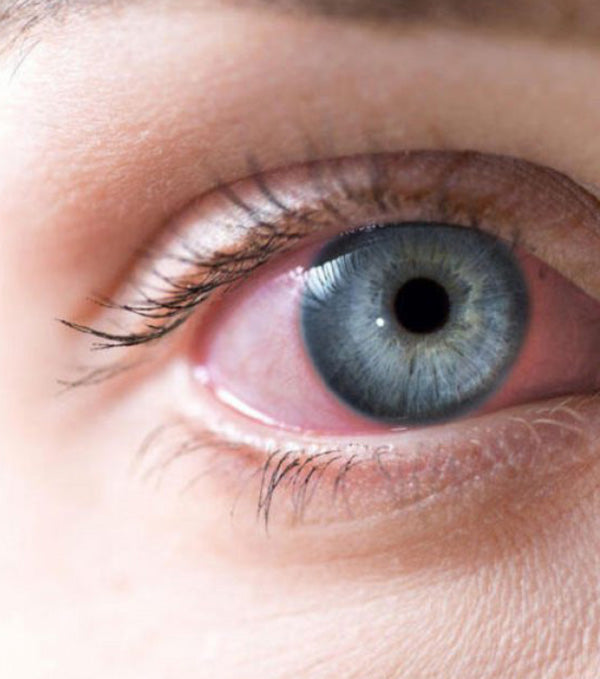
Tears may contain viral particles. The adenovirus, in particular, that results in a highly contagious conjunctivitis, can be spread through contact with an infected person’s tears. Evaluation of the novel coronavirus and its transmission has shown that there’s a possibility of the presence of SARS-CoV-2 in the tears and conjunctival secretions of patients who have a positive conjunctivitis infection. Patients who have SARS-CoV-2 but no conjunctivitis have been shown to be at low risk of transmitting the virus through tears or conjunctival secretions2. Blood borne infections pose little threat in the optometric practice, unless there are any spills of blood or other body fluids, which is largely uncommon.
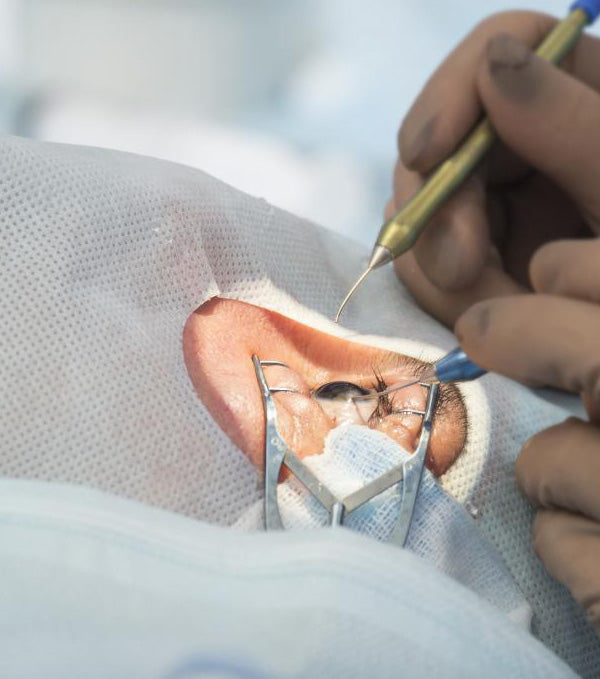
Needles and other sharps used in optometric practice pose the risk of needle-stick injuries, which increases the risk of transmission of infection.
Patients who have dry eye syndrome, or who present with inadequate lid closure may be more susceptible to infection, as are those patients with other risk factors such as lower immunity, malnutrition, comorbidities or those in higher age groups (3).
INDIRECT TRANSMISSION:
An inert object may be a source of microorganisms and pose a risk of cross-transmission of infection. Common objects or agents used in ophthalmic practice that may harbor infection include:

Contact lenses, their cases and other items related to contact lens wearing
Devices and equipment used in practice such as:
a. Tonometer heads
b. Pachymeter
c. Diagnostic lenses
d. Trial frames
e. Chin and forehead rests
f. Refractor heads
g. Rulers
Surfaces such as flat surfaces in the environment of the practice, those that harbor dust, door handles, chairs, displays, phones, computers etc.
MAJOR RISKS OF INFECTION
There is a wide diversity of microorganisms that inhabit the areas of the periocular region. Staphylococcus aureus, coagulase-negative Staphylococcus spp., Propionibacterium acnes, and coryneform bacteria have been identified as the most common. Many of the species of bacteria, fungi and viruses are harmless, or they provide a level of protection against other more pathogenic organisms.
These pathogenic organisms, in particular bacteria, contribute to the majority of eye infections, with some of them being resistant to treatment of antibiotics4. Most of the infections occur as a result of transmission of infection from the tissues that make up the sinuses, which account for 60-70% of cases. However, direct injury, pre-existing conditions that affect the orbital region and haematogenous spread are also associated with infections.
Common infections that may be seen in optometry are:
• Bacterial conjunctivitis. Usually caused by Staphylococcus aureus.
• Viral conjunctivitis. Usually caused by Adenovirus.
• Other viral infections such as herpes simplex, varicella zoster, molluscum contagiosum, measles and mumps, all of which can cause conjunctivitis.
• Blepharitis, which causes inflammation of the eyelids and is associated with staphylococci and propionibacteria.
ORBITAL INFECTIONS: AETIOLOGY
Infection from neighbouring structures
• Nasal and sinus disease
• Sinus or lacrimal sac mucocoele
• Orbital fracture
Direct injury
• Minor surface injuries (e.g. scratches) which can lead to necrotising infections
• Penetrating orbital or sinus injury
• Iatrogenic: surgery for eyelid, orbital or sinus disease
Pre-existing orbital disease (e.g., dermoid with a fistula to the the skin)
Haematogenous spread
• Opportunistic infections in a immunocompromised individual • Dental infection
• Subacute bacterial endocarditis
Reference: Hamed-Azzam, S., et al. Common Orbital Infections ~ State of the Art ~ Part I. J Ophthalmic Vis Res. 2018 Apr-Jun; 13(2): 175–182.
EYE INFECTION CHAIN OF TRANSMISSION
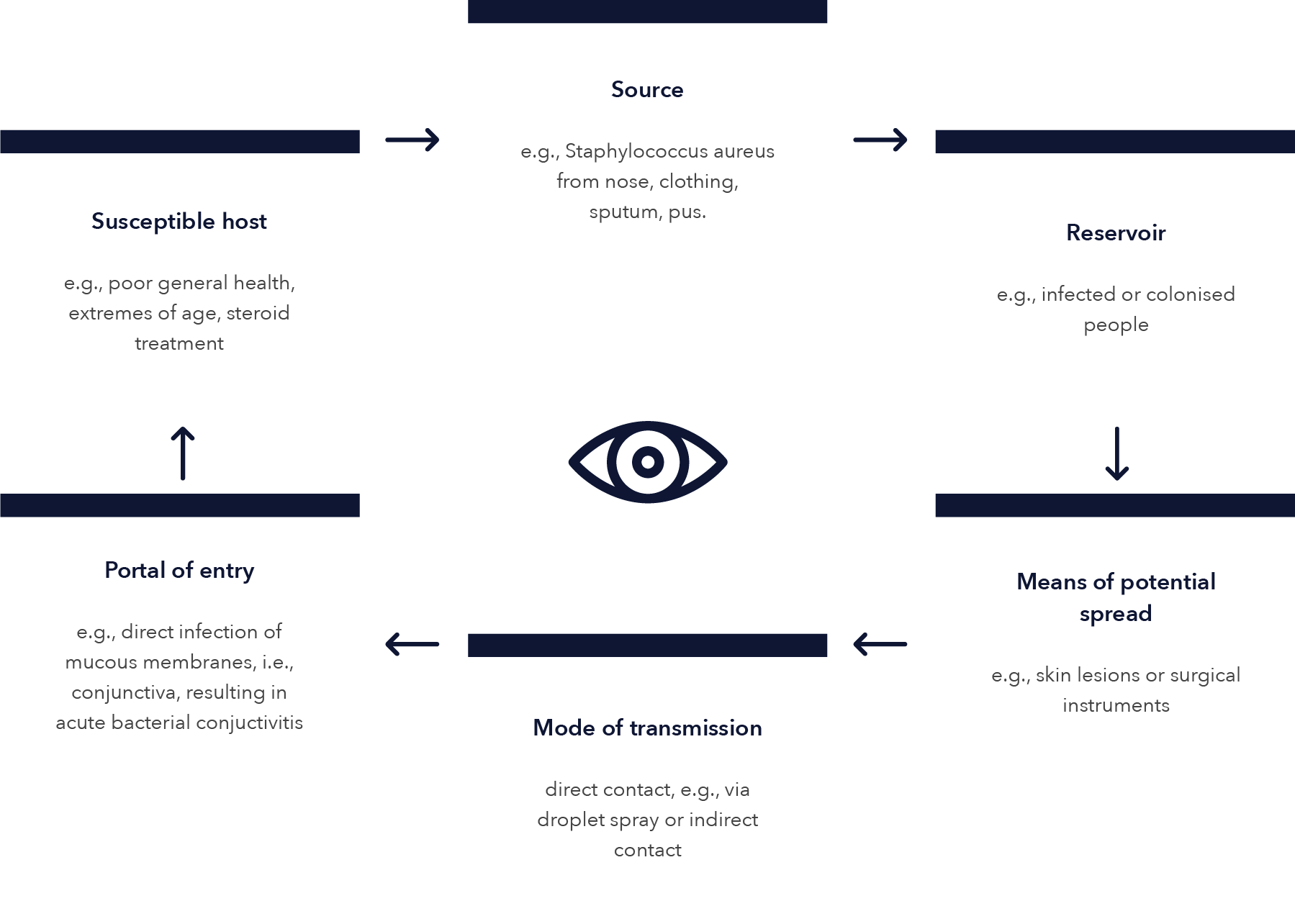
Reference: Seewoodhary, R., & Stevens, S. Transmission and Control of Infection in Ophthalmic Practice. Community Eye Health. 1999; 12(30): 25–28.
Serious infections may lead to loss of vision, which occurs in more than 10% of patients5. There is also a risk of meningitis, intracranial abscess formation and death as a result of serious infections. For this reason, it is essential to promptly manage any orbital infection to reduce the risk of complications.
COVID-19 AWARENESS IN OPTOMETRY PRACTICES
In March of 2020, the American Optometric Association’s Health Policy Institute (HPI) issued an updated statement regarding the risk of infection of the novel coronavirus, pertaining to eye health and vision care in the community6. The statement encouraged every optometrist to familiarise themselves with the risks associated with the virus and how it may affect optometry practice.
While viral conjunctivitis is typically caused by the adenovirus, photophobia, irritation of the conjunctiva as well as signs of infection thereof which may or may not include watery discharge, may be ocular signs and symptoms of COVID-19.

In any case of suspected infection of a patient, optometrists, healthcare providers and optometry technicians need to have a clear understanding of the measures that need to be in place to prevent the spread and cross-transmission of the infection. Along with thorough handwashing, using the correct PPE and ensuring regular disinfection of equipment and high touch surfaces, there are a number of ways technology can help to reduce or even eliminate the risk of transmission in everyday optometry practice.
EYECARE AND GENERAL WOUND CARE
Eye infections are easily spread, and infection control is essential not only to prevent possible cross-transmission in the optometry practice, but to prevent community spread while the patient is in their home environment.
Briotech, which is made of a hypochlorous acid (HOCl) solution, has been used in wound care, dermatology and dentistry, and also has an important role in eye care7.
HOCl has the same active ingredient in bleach, with one significant difference: it has a different chemical structure, which makes HCOl completely safe for direct contact with the skin and mucous membranes, whereas bleach would cause serious and debilitating injury.
Briotech is a completely natural antimicrobial agent that is produced by the human body. It is produced in response to an immune reaction and has been shown to kill microorganisms and neutralize toxins on contact. While potent, Briotech is non-toxic and gentle even with daily use, which offers an appealing application to infection control and treatment in optometry.
HCOl has been shown to provide relief from dry eyes and hordeola, and is an effective treatment option for the symptoms associated with blepharitis and meibomian gland dysfunction, or MGD, where it not only reduces bacterial load, it promotes secretions (8).
Briotech is safe to use alongside frequent surfactant cleaners, where the combined therapy with HOCl has been shown to improve outcomes of more severe conditions.

Other wounds also pose a threat of infection in the optometry offices. A healthcare worker who may have a suspected infection should not come into direct contact with a patient. If a healthcare worker or technician has an infected or potentially infected lesion on their exposed skin, recommendations are that it is to remain covered with an occlusive dressing. Briotech offers additional protection for the spread of potential infections from the site, with the solution being sprayed directly onto the wound to kill microorganisms.
HCOl has been shown to decrease bacterial load by more than 90%, without any detrimental effect or significant alterations to the normal microbial diversity of the human microbiome9.
OBJECT DISINFECTION
Some optometric devices may not withstand traditional disinfection methods, however, need to be disinfected to protect vulnerable patients and prevent the spread of infections. With the UV Smart, objects such as the N95 facemask, mobile phones and other electronic devices have been disinfected using the UV Smart, with no traceable negative effects to their aesthetics or function.

The UV smart device offers log-5 UV-C disinfection in as little as 25 seconds using fast cycle, Impelux technology that ensures consistent and repeatable disinfection. Using UV light as the disinfecting agent for non-critical and semi-critical medical instruments and equipment, the closed contained is user-friendly, safe to use and emits no outward radiation. Once the object is disinfected from pathogens such as bacteria, viruses, spores, fungi and yeasts, the lid opens automatically.
Additionally, Briotech (HOCl solution) may also be used to quickly clean instruments such as tonometry tips.
AEROSOL CONTROL AND SURFACE DISINFECTION
Any surface area in the environment of a practice may be a source of transmission of infection. Inert objects and agents need to be regularly and thoroughly cleaned in order to reduce the risk of infection.
Aerosols may settle on surfaces and, depending on the type of microorganisms, may survive for a long period of time, posing the risk of transmission of an infection as they easily contaminate a person’s hands when they come into contact with them.
While good hand hygiene, thorough and regular cleaning as well as decontamination and disinfection of surface areas is essential, it may not be sufficient to completely rule out the possibility of cross-transmission of infection in the optometric practice.

The process of decontamination is typically performed at one of these three levels depending on the item that requires decontamination:
Remove visible debris from the object using a detergent solution.

Use heat or chemicals to disinfect and reduce microorganisms on the surface of the object.

Sterilization to remove microorganisms including spores. In optometric practices, true sterilization of equipment and objects is not often needed.
CASPR technology offers a true no-touch solution to surface cleaning and addresses some of the major concerns around their cleaning and disinfection in the optometry practice:
• Provides continuous disinfection
• Facility-wide coverage
• Eliminates human errors in cleaning techniques
• Reduced the need for additional resources and additional capital expenditure
• Addresses ignored, hard-to-reach and impractical areas
• Safe to use continuously in occupied spaces
• No sensitive use parameters to consider
Using a natural catalytic converter, which is installed directly into the HVAC system, the CAPSR can be operated 24/7 without any training, while blanketing all surfaces leading to a proven reduction in CFUs by up to 99.9%.
The device works by converting air and water into oxidizing molecules, which have proven action and effectiveness against a broad spectrum of clinical pathogens that may be present on any exposed surfaces.
Tested in a hospital setting, use of the CAPSR device significantly reduced staff absenteeism while maintaining almost complete elimination of microbial burden in hospital rooms, nursing stations and on targeted textiles in the hospital environment.

Aerosols, which are small airborne particles, are created during heavy breathing, talking loudly, coughing and sneezing, and may contain contaminated particles that can transmit infections. Small droplets may remain airborne for long periods of time, particularly when they are less than 0.1μm. Research conducted in Japan, using high sensitivity cameras, has shown that these tiny droplets created by taking, may remain in suspension for 20 minutes or longer (10). The exposure risk of anyone entering that environment within this timeframe therefore increases substantially, even if they do not ever come into direct contact with the infected person.
These small droplets, as well as larger ones, may pose additional problems for the risk of contamination. They may fall onto surfaces in the direct vicinity of the infected person, or they may float in the air and contaminate surfaces in a range of 1 meter from where the exposure took place (11). Again, persons that come into contact with these contaminated surfaces may become infected without exposure to an infected person.
Air purification systems may be one of the best measures to reduce the risk of cross-transmission that aerosols create.
The Light Progress, which uses UV light and titanium oxidation technology, offers an all-in-one air purification, sterilization and decontamination system.
Easy to install, this device consists of a fan that continuously draws in contaminated air, flowing it through a UV-C filtration box, which effectively eliminates all microorganisms in the air particles within minutes. Additionally, the internal titanium oxide germicidal box degrades debris formed during decontamination of organic matter, which purifies the air and neutralizes odours.
UV-C technology has been used by many world class organizations to purify water, environments and air conditioning systems for decades. It is a reliable technology that has been shown to be highly effective for eliminating 99.9% airborne infections, including the novel coronavirus. Commonly used UV-C devices may pose multiple problems. They may be dangerous to use in occupied spaces due to the exposed UV radiation and they may need to be moved to target certain surfaces. The Light Progress, which is a contained device that combines a fan and UV-C device, offers a no-touch, continuously operational system that draws in potentially contaminated air, filtering out harmful particles and then exhausting clean and purified air back into the environment.
Alcohol wipes should not be used as a sole method of decontaminating high-touch surfaces or devices. Alcohol has been shown to fix prion proteins to smooth surfaces.
With Briotech, not only has it been shown to effectively inactivate infectious microorganisms including bacteria, fungi, viruses and spores, it has a high safety profile for use on sensitive tissues, including being an effective disinfectant solution that can be used directly on mucous membranes, which is important should the solution accidentally come into contact with a physician/technician or patient’s eye.

Additional methods of reducing infection transmission (12):
• Careful screening of patients asking appropriate questions related to high risk groups.
• Effective hand hygiene and PPE use. If you come into contact with a patient with a known infection:
• Avoid wearing jewelry and wristwatches
• Keep nails short, clear and clean
• Cough and sneezing etiquette
• Using aseptic, no-touch techniques were possible
• Safe and appropriate disposal of single-use equipment, sharps and waste
CONCLUSION
Infection control in optometry practice is a critical part of every day operations. While threats of cross-transmission may not appear as high risk as healthcare fields that perform exposure-prone or invasive procedures, there are a vast number of factors in optometry practice to consider. Putting the appropriate measures in place as mentioned above may have a dramatic impact on the risk of cross-contamination of infection and exposure to microorganisms that may be present in optometry practice.

REFERENCES:
1. Lian, K., et al. Infection control guidelines for optometrists 2016. Clinical and Experimental Optometry. 2017. 100(4).
2. Xia, J., et al. Evaluation of coronavirus in tears and conjunctival secretions of patients with SARS‐CoV‐2 infection. Journal of Medical Virology. 2020. 92(6).
3. Ayliffe G. Infection Control, Nosocomial Infection, Problems and Organisation. Africa Health. 1996;18:9–10.
4. Mohammed, A.A., Ali, M.M. & Zenebe, M.H. Bacterial etiology of ocular and periocular infections, antimicrobial susceptibility profile and associated factors among patients attending eye unit of Shashemene comprehensive specialized hospital, Shashemene, Ethiopia. BMC Ophthalmol 20, 124 (2020).
5. Tomaç S, Turgut S. Orbital cellulitis and irreversible visual loss owing to acute sinusitis. Ann Ophthalmol (Skokie). 2006 Summer; 38(2):131-3.
6. American Optometric Association’s Health Policy Institute (HPI). Statement: Doctors of Optometry and COVID-19. 2020.
7. Wang L, Bassiri M, Najafi R, et al. Hypochlorous acid as a potential wound care agent: part I. Stabilized hypochlorous acid: a component of the inorganic armamen- tarium of innate immunity. J Burns Wounds. 2007;6:e5.
8. Harsch AG, Stout N, Lighthizer N. Beat the blepharitis blues. Rev Cornea Contact Lens. 2016;153(7):12-15.
9. Ono T, Yamashita K, Murayama T, Sato T. Microbicidal effect of weak acid hypochlorous solution on various microorganisms. Biocontrol Science. 2012;17(3):129-33.
10. Stadnytski, V., et al. The airborne lifetime of small speech droplets and their potential importance in SARS-CoV-2 transmission. PNAS. 2020.
11. Barker J, Jones MV. The potential spread of infection caused by aerosol contamination of surfaces after flushing a domestic toilet. J Appl Microbiol. 2005;99(2):339–47. Pmid:16033465
12. NHMRC. Australian Guidelines for the Prevention and Control of Infection in Healthcare. Commonwealth of Australia. 2010.