Anyone who is in constant close contact with other people, is at risk of contracting an infection as a result of exposure to various microorganisms that another person might expose them to. While considered a rare occurrence, both patients and their dentists are at risk of acquiring and transferring diseases as the practices present multiple opportunities that allow for cross-contamination. These risks cannot be considered completely negligible, particularly when there is a breakdown of basic infection control and precautions are not meticulously followed.
With daily practices in dentistry involving working with sharp and high-speed instruments, there is constant risk of exposure to microorganism cross-transmission. In addition, the oral cavity presents a unique environment that promotes the growth of microorganisms due to moisture and temperature as well as the provision of metabolites that allow them to flourish.
With the rise in risk of infection as a result of infectious diseases, dentists have begun using protectors for their eyes, nose and mouth; a practice that was not common for the most part of the last century. Contamination in healthcare staff has increased on average 5.84% year on year, reports the World Health Organization (1), which has encouraged the standardization of the use of personal protective equipment.
Studies have revealed a higher prevalence of influenza virus, respiratory syncytial and adenovirus antibodies among dentists when compared to non-dental practitioners (2).

Most commonly, precautions are taken to prevent infection spread via exposure to blood and other body fluids. Saliva poses one of the biggest problems, where it may spread microorganisms due to contamination with blood or gingival fluid. The risk of exposure to infection should also be considered when both patient or practitioner come into contact with contaminated objects that may have been exposed to large microorganism-containing respiratory droplets. Surface contact may pose as great a risk of cross-transmission to both practitioner and patients (3).
In a UK study, settle plates were used to determine the presence of airborne microbes in a dental clinic. Data collected during clinical activity revealed the presence of 193 distinct colony morphotypes that comprised 73 species. Astonishingly, 48% percent of those were found to be resistant to at least one type of antibiotic (4).
Risk of disease spread increases when there is a breakdown of infection control practices.
FAILURE OF INFECTION CONTROL PRACTICES
Some of the most common incidents occur when there is a break down in basic infection prevention procedures, which include unsafe injection practices, failure to sufficiently sterilize equipment between patients and failure to correctly monitor autoclave procedures.
The recommended checklist as provided by the Centers for Disease Control and Prevention in the Guidelines for Infection Control in Dental Health-Care Settings, the Summary of Infection Prevention Practices in Dental Settings: Basic Expectations for Safe Care, provides clear instructions that allow for evaluation of compliance in dental care settings (5).
Standard precautions for infection prevention include:
• Hand hygiene.
• Use of personal protective equipment (e.g., gloves, masks, eye-wear).
• Respiratory hygiene/cough etiquette.
• Sharps safety (engineering and work practice controls).
• Safe injection practices (i.e., aseptic technique for parenteral medications).
• Sterile instruments and devices.
• Clean and disinfected environmental surfaces
As these practices alone are unable to fully prevent transmissions, they are to be supplemented with transmission-based precautions. These transmission-based precautions are intended to prevent the spread of disease, particularly when patients are suspected to have an active infection and there is a higher risk of transmission through skin contact, sneezing, and coughing for example. Contact, droplet and airborne precautions as stipulated by the hospital and other ambulatory services should be followed in these instances (6).
COMMON CROSS-CONTAMINATION INFECTIONS FACED IN THE DENTAL SETTING
Three main factors need to be considered to determine the relative risk of cross-transmission and infection.
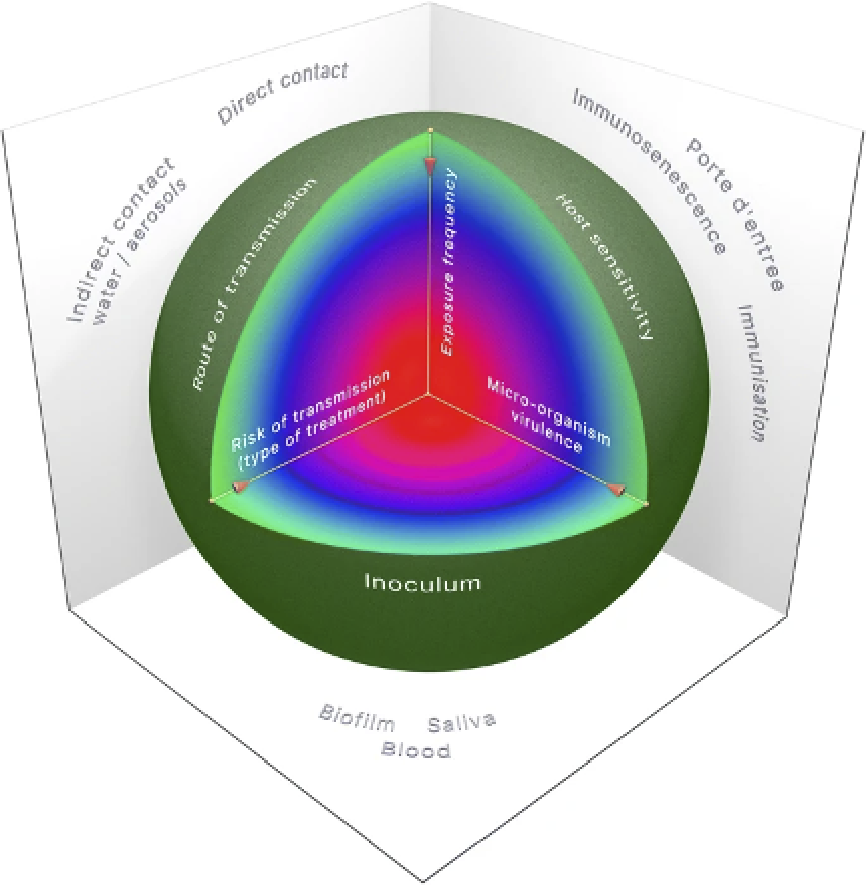
Figure 1: Considerations of risk of infection in dental offices (7)
There are two other important modes of infection that need to be as cautiously considered in dental offices: droplet and airborne infections.
Droplet infection is most common when a patient coughs, sneezes or talks loudly, allowing for the transmission of infected particles to be directly spread to the clinician who may not be wearing the appropriate PPE.
These droplet transmission and airborne infections are now as important to take into account.Airborne infection, while similar in the mode of transmission, involves smaller particles of infected particles to be inhaled as they remain in the air for a longer period of time (8). A clinician may sneeze before a patient enters their room, and the patient then breathes them in, minutes later. Additionally, the PPE a clinical wear needs to be considered when it comes to airborne infections. A standard surgical mask does not have a filtration system that filters out micro particles, thus, if a patient is suspected of being infected with an airborne disease, an N-95 respirator is required.
AIRBORNE TRANSMISSION
Airborne infections are transmitted via droplets that contain microorganisms. When aerosols, which are named droplet nuclei when they are between 1–5μm in size, and droplets when greater than 5μm in size, are liquid or solid particles that may become suspended in the air. When they contain any kind of organism, they are referred to as bio-aerosols. Bio-aerosols differ depending on their environment, which includes changes in humidity, air flow and temperature.
Droplet nuclei offer the greatest risk of transmission as they may be suspended in the air for hours, they can be transported in the air over long distances and when they settle, they contaminate surfaces (9). Droplets, on the other hand, are typically quicker to fall and settle, but it has been proven that they can contaminate surfaces in the range of 1 meter from where first exposure took place (10) When inhaled, these different types of droplets are capable of infecting a host as a result of penetrating deep into the alveoli of the lungs.
According to a study, transmission the most common viruses and bacteria that are transmitted via dental water and aerosols are:
• Cytomegalovirus
• Measles
• Mumps
• Influenza, rhinovirus and adenovirus
• Rubella
• Streptococcus pyogenes
• Mycobacterium tuberculosis
• Legionella pneumophila
• Pseudomonas aeruginosa
Dental water lines pose a unique problem in that contamination can occur from water that is used in the line itself or from backfiring of water that is being used on the patient during a procedure. When the water line is contaminated, the moist environment, the controlled room temperature and the high surface adherence of the water line is an ideal surface for biofilms to develop. Both the clinician and patient are then frequently exposed to the contaminated water via direct transmission through splatter and drinking or via indirect transmission, through aerosols produced during the procedures (11).
Aerosols can also be spread through the dental office via talking, coughing and sneezing, and it is these aerosols that are the most common cause of infection such as the common cold, influenza and other respiratory infections such as TB and legionnaire’s disease.
Studies into the various types of coronavirus have yielded some evidence to suggest the risk of infection. While research is continuing, and experimental evidence needs to be conducted and reproduced, we may have an idea of how infection can occur.
In general, infection of coronavirus could occur through exposure to around 1,000 particles infected with the virus. When it is considered that a cough releases around 3,000 droplets, a sneeze, 30,000 droplets and a single breath depending on whether it is forcefully exhaled or not around 50 to 5,000 droplets, we have a better idea of why it is so important to reduce the exposure to these droplets.
New research published about the transmission routes of CoV-2 and the controls needed to be put into place in dental practices suggests that dental professionals play an important role in preventing transmission and that infection control is one of the most essential practices in dental offices to reduce the risk of person-to-person infection.
While it has been suggested that the novel coronavirus may be spread via aerosols formed during medical procedures, further research needs to be conducted in this regard (12). It has, however, been confirmed that the virus may also be spread through contact with asymptomatic patients (13), and thus, even thorough screening of patients to elucidate their symptoms as a means to base the decision on which to proceed with the dental procedures, may be limited in their effectiveness.
When we consider the particle size of the droplets, as was discussed earlier in this article, we have an idea of just how long these smaller particles remain airborne and in circulation in a closed, poorly ventilated area. Research conducted in Japan shows that even during a conversation, held at normal standing distance, 0.1μm droplets are detected using high sensitivity cameras. These droplets don’t float away and may remain in suspension for longer than 20 minutes (14).
Another test that collected data which consisted of 10 experimental conditions, and the comparison of CoV-1 and CoV-2, shows that Cov-2 micro-droplets may remain suspended in the air for 3-4 hours in poorly ventilated spaces.
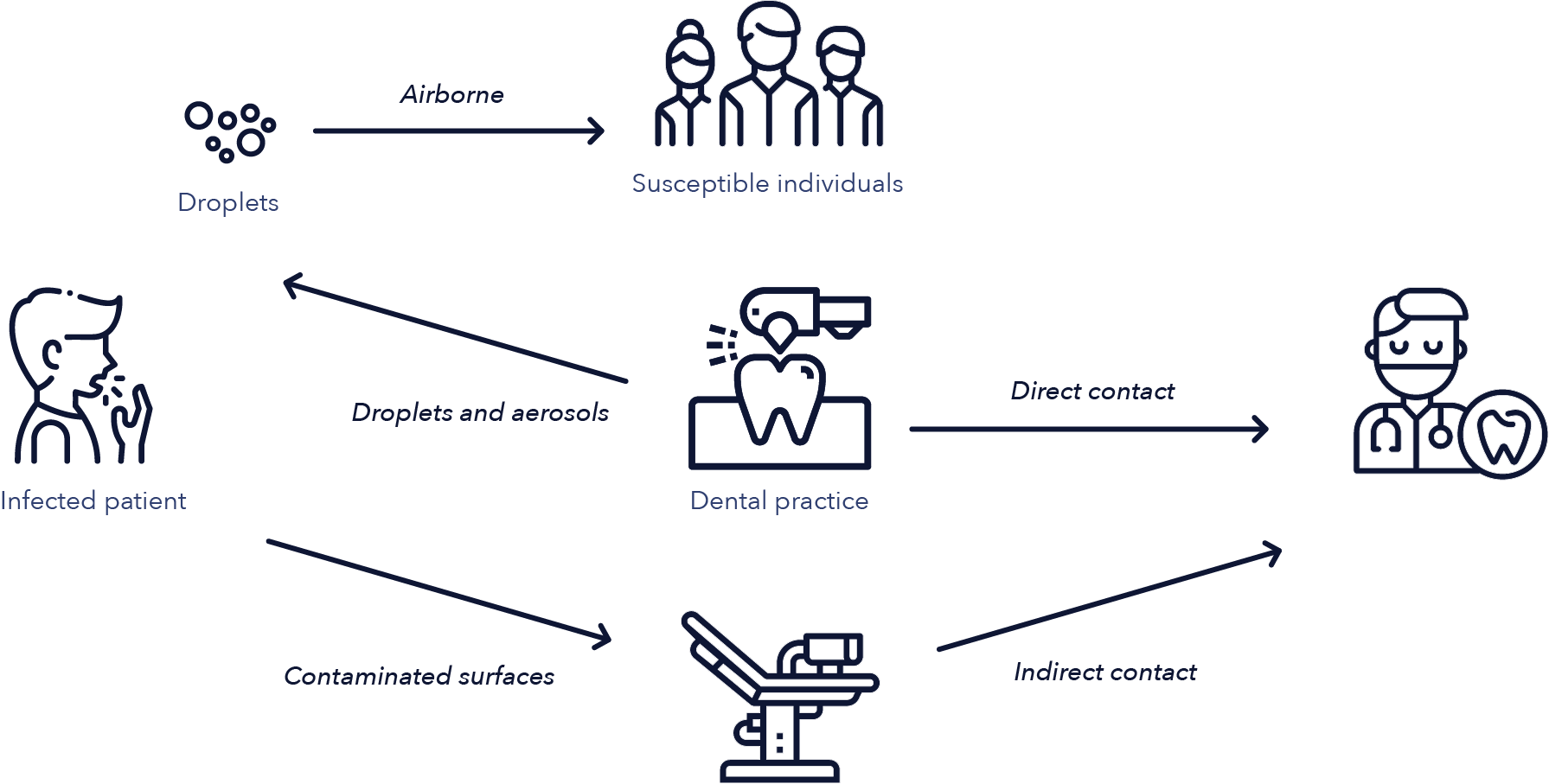
Figure 2: Routes of transmission of airborne microorganisms in dental offices (15)
Additionally, CoV-2 was shown to be more stable on plastic and stainless steel surfaces, and that viable viral particles were detectable on these surfaces 72 hours after application. The virulence of the infected materials was, however, greatly reduced (16).
Much of the routes of transmission and infection risk related to the novel coronavirus have been compared to previous pandemic viral outbreaks, such as that which occurred during the SARS-CoV-1 outbreak. Due to the similarities in the mechanism of action of the CoV-2 and CoV-1, which targets the ACE2 receptors in the respiratory tract, infection control has largely been based on what has previously been found to be effective in this regard (17).
One way to do so is to consider the air quality in dental offices and how improvement in air circulation may reduce the risk of infection that is spread through bio-aerosols.
TECHNOLOGICAL ADVANCES FOR MANAGEMENT OF INFECTION RISKS IN DENTAL OFFICES
AIR PURIFICATION
From hospitals, to daycare centers, and even the common areas used by the Toronto Raptors, Maple Leafs, and Blue Jays, the Montreal Canadiens, and Vancouver Canucks, air purification systems are in place to provide surgically clean and purified air due to the high risk of airborne microorganism transmission in these environments, and the devastation an infection may cause.
Indoor air quality and the contamination of confined, low ventilated spaces has been well documented to reduce air quality in dental practices. However, it appears that many dental offices have not as yet adopted these types of practices. As a result of anxiety associated with dental procedures, the environment is well thought of to provide a sense of calm to the incoming patient, however, air quality and risk of infection should also be made a top priority.
Poor air quality and low ventilation in addition to the redistribution and recycling of poor quality air by HVAC systems, also has an effect on the clinician’s performance. Research shows that superior air quality enhances performance not only due to the reduced incidence of headaches and dry, irritated eyes, nose and throat, but because of the positive effect it has on the risk of contracting a respiratory illness. , and even the common areas used by the Toronto Raptors, Maple Leafs, and Blue Jays, the Montreal Canadiens, and Vancouver Canucks, air purification systems are in place to provide surgically clean and purified air due to the high risk of airborne microorganism transmission in these environments, and the devastation an infection may cause.
Air filtration systems such as the Light Progress work by continuously drawing in air, filtering out harmful particles and exhausting clean air back into the environment. In addition to filtration, due to the risk of viral infections, the addition of a UV device, installed into the filtration system may help to further purify the air.
Studies to determine the effectiveness of filtration/UV combined fans in closed rooms and offices considered to be environments of normal working activity.
Devices such as these have been shown to be successful in destroying microbial load almost in totality, with results suggesting eradication of up to 99% in filtered air.
Additionally, fungal load revealed the same results, which provides a remarkable intervention for reducing the risk of infections contracted via airborne transmission.
The UV fan air purification device uses a fan to convey air from the external room to the inside of a germicidal box, which decontaminates the air from microbes and chemical compounds. The filters that make up the germicidal box contain titanium dioxide, which degrades organic and inorganic matter, and destroys the membranes of microorganisms. Toxic odors and noxious gasses such as ammonia and sulfur are commonly released due to degradation of organic matter. These odours, which may cause discomfort to those in the area of the device are absorbed.
The germicidal box also contains UV-C lamps, which improves the germicidal power due to the reflection that the lamps provide. Within seconds, the air is then introduced back into the environment, filtered and without contaminants.
This air purification, toxin filtration and UV-C combination system has been shown to be effective against common airborne illnesses such as Avian Flu Virus (H5N1), SARS, Influenza, Herpes, Legionella pneumophila, TB and also infections that may develop as a result of yeasts, molds and fungi that may be inhaled from the air.
With this information on hand, we can see that it is highly possible to improve air quality in confined spaces using filtration and UV-C devices. In dental offices, where 40-50 patients may be seen on any given day, reducing the risk of airborne illnesses should be top priority.
SURFACE DISINFECTION
In the 1970’s, a landmark infection-control study, published by Dr James Crawford, showed just how significant cross-contamination of the dental office can be when looking at salvia spray alone. In the experiment called “if saliva were red”, practitioners went about their normal clinical procedures using mannequin patients, after having dipped their fingers in red poster paint. The results demonstrated the possible contamination of salvia-covered practitioner fingers across the work area. Years later, a number of studies have expanded on the concept, showing that secretions during dental procedures are a great source of cross-contamination between practitioner and patient, with inanimate surfaces being of the greatest concern (18).
It is for this reason that surface disinfection is another essential consideration in dental practices. Even with the best of intentions and attention to detail, however, cleaning agents may not be able to thoroughly disinfect surfaces that are considered high-touch areas in dental offices.
Two main approaches are used to reduce the risk of cross-contamination via surfaces: pre-cleaning and disinfection, and single-use barrier protection.
Where the most common approach to infection involves surfaces pre-cleaning and disinfection, not all surfaces in dental offices can be pre-cleaned. Multiple surfaces such as buttons, drawer pulls, control switches and other smaller more intricate surfaces typically need to be covered with a barrier to reduce the risk of contamination.
Surface pre-cleaning and disinfection can be expensive, in terms of staffing requirements, and time consuming. The spray-wipe spray technique suggests that surfaces need to be wiped down before disinfecting agents can be applied. The disinfecting agent then needs to be allowed to settle on the surfaces for 5-10 minutes before being wiped again with a paper towel. Additionally, the surfaces throughout the dental offices may only be cleaned periodically, which allows a significant opportunity for cross-contamination to occur as a result of high volume movement throughout the practices.
Barrier protection may appear to be a simpler, less expensive and as effective a way to reduce risk of infection, but it too is not without its disadvantages. They too may need to be cleaned or frequently changed (19).

One solution to current failures of adequate aseptic techniques comes in the form of an easy-to-install device that fits right into the HVAC system. Called the CASPR, this true ‘no touch’ device has been shown to address multiple gaps in current cleaning and disinfection protocols. Not only does its installation into the HVAC allow for it to operate 24/7, facility-wide, it continuously blankets all surfaces with proven effectiveness to reduce CFUs by 99.9%. The photocatalytic device converts air and water molecules from the HVAC into oxidizing molecules, which have been proven to have an effect against a broad spectrum of clinical pathogens. Due to the oxidation of microorganisms, the CASPR has also been shown to eliminate odors, which may provide significant benefit in dental offices.
The effects of the oxidation process are non-toxic to humans, and the oxidizing molecules have little effect on the surface they settle on, which means that CASPR is safe to use even in occupied spaces, on a continuous basis.
Tests conducted in hospital settings in Oklahoma and Iowa have shown significant reduction in surface infections; more than 90% reduction of average fungal and MRSA contamination and more than 98% reduction in overall bacterial contamination has been found on high-touch surfaces. Additionally, the use of the CASPR system in hospitals improved room turnover rates between patient admissions, and reduced staff absenteeism by 42%.
CASPR eliminates the risk of physical cleaning errors, reduces the need for disinfection agents and improves the overall environment with regards to infection risk on dental offices.
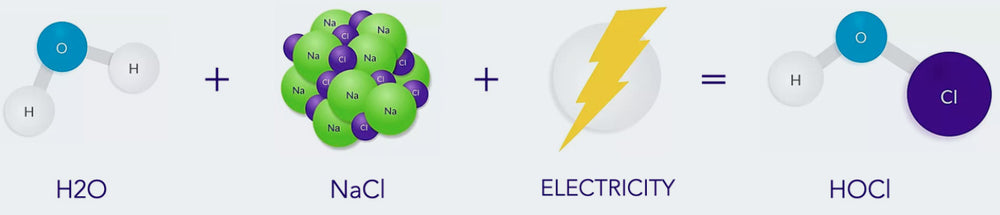
A hypochlorous acid-based (HOCl) disinfectant spray is yet another way to quickly and effectively clean an environment. HOCl has long been investigated as an alternative to alcohol-based solution as a disinfectant. Until now, previous attempts at creating an HOCl solution have proven unsuccessful, resulting in a toxic, unusable agent. BrioHOCl has overcome the previous challenges, and offers a safe, effective and easy-to-use solution.
Misting or fogging an area with the Briotech spray quickly neutralizes viruses, bacteria, endospores, fungi and prions within 5 contact minutes. The BrioHOCl degrades to water with a low NaCl concentration within minutes, leaving no residue or hazardous materials behind. This technologically advanced form of HOCl is low risk for both patients and practitioners, with the solution having been shown to be safe for use directly on open wounds and mucous membranes.
The Briotech solution may also be used for personal surface cleaning. The solution can be sprayed directly onto the hands for disinfection purposes, further reducing the risk of cross-contamination of high touch surfaces.
LINE CLEANING AND ORAL RINSE
Briotech, along with its use in surface and air disinfection, can be utilized as a solution to clean and disinfect dental water lines and as a pre-procedure dental rinse in patients who have or who are suspected of having periodontal diseases (20).
Dental water lines can become heavily colonized with waterborne microorganisms. Findings have shown that bacteria, fungi and protozoa, which are known to form biofilms (21) on the internal surfaces of the water lines, are a source of contamination and possible route of infection in dental practices. While these microorganisms are often not harmful or dangerous to the general population, care needs to be taken to prevent risk to susceptible patients.
There have also been reports that have offered information pertaining to possible pathogenic organisms contaminating dental water lines, with the risk of causing fever, inflammation and even resulting in septic shock in rare cases. Cleaning and disinfection of these water lines is thus pertinent to dental procedures, whether there is a low suspicion of serious contamination or not; the risk is there.

Guidelines for the cleaning of water lines suggest that they be flushed with water at the start of every workday for at least 2-3 minutes with water. This procedure, however, will not address biofilm risk, which is where Briotech comes in.
Due to the effectiveness of a hypochlorous acid having been shown to have a significant impact on microorganism function, flushing dental water lines with Briotech effectively clears biofilms and sterilizes the water that is then able to be safely used during dental procedures. Briotech can also be effective in cleaning and disinfecting the handpieces attached to these waterlines (22).
Briotech has also been found to be effective as an oral rinse.
A 2018 study, that compared an HOCl solution with a chlorhexidine (CHX) solution as a mouth rinse in dental practice showed that the HOCl mouthwash had a significant effect on the O’Leary index, Snyder test, bacterial motility, and Filamentous species when compared to the CHX and saline mouth rinse solutions.
Briotech, with its high safety profile and use on even sensitive conditions, offers a valuable alternative to current use of CHX, which has been said to have a strong fragrance and taste, which is offensive to many dental patients (23).
PPE REUSE
In our current environment, with the COVID pandemic, healthcare workers have expressed serious concerns around the availability of PPE. With the requirements for dental practitioners and staff including the use of appropriate PPE going forward, the use of N95 face masks may increase in the dental industry.

While these masks are designed for single use, the UV Smart device offers the solution of disinfecting these masks when needed, provided they are not visibly soiled.
The device, which is a closed container that emits UV light using Impelux technology onto any item placed into the container, has been shown to sterilize and disinfect in 25-100 seconds. UV light has for a significant time been used to effectively kill and disinfect the clinical environment. UV-C has been used since the 1900s to disinfect water, and is currently widely used in air disinfection.
The process of using UV-C to inhibit microorganisms has since been shown to be an effective way to eliminate SARS CoV1, which is part of the family of coronaviruses.
The UV Smart device can also be used to disinfect non-critical and semi-critical dental tools. They can be placed directly into the container, which provides 5log disinfection (99.99% or 1 in 100,000 survival rate, which is known as high-level disinfection), reducing the risk of cross-contamination should these devices not be cleaned thoroughly through manual disinfection techniques.
The process that the UV smart device uses has no detrimental effects to any of the materials of these items, and due to the emission of UV light in a closed container, it poses none of the drawbacks of current exposed UV-C devices .
CONCLUSION
Disease prevention has been proven to be more cost effective than curing a disease (24). It is thus of utmost importance to infection control to continue to maintain high standards in preventative measures undertaken in the dental office setting.
While current guidelines for precautions that significantly reduce direct and indirect contact to microorganisms needs to continue, with the increase in risk of droplet transmission and airborne infections, which also increases the risk of surface cross-contamination, further precautions may be warranted. In healthcare associated infections, the burden for both the patient and the clinician needs to be considered in terms of their environment, and the overall risk of infection in the dental office setting needs to be evaluated as a means to put into place the most appropriate interventions to significantly reduce the risk of disease cross-transmission.
REFERENCES:
1. Geneva: WHO; 2002. World Health R. Reducing Risks, Promoting Healthy Life; pp. 1–167.
2. McCarthy, G. Risk of Transmission of Viruses in the Dental Office. J Can Dent Assoc 2000; 66:554-5, 557
3. Szymańska J. Microbiological risk factors in dentistry. Current status of knowledge. Ann Agric Environ Med. 2005;12(2):157‐163.
4. Decraene V, Ready D, Pratten J, Wilson M. Air-borne microbial contamination of surfaces in a UK dental clinic. J Gen Appl Microbiol. 2008;54:195–203.
5. Centers for Disease Control and Prevention. Summary of Infection Prevention Practices in Dental Settings: Basic Expectations for Safe Care. Atlanta, GA: Centers for Disease Control and Prevention, US Dept of Health and Human Services; October 2016.
6. Australian Guidelines for the Prevention and Control of Infection in Healthcare. (2019)
7. Volgenant, C.M.C., de Soet, J.J. Cross-transmission in the Dental Office: Does This Make You Ill?. Curr Oral Health Rep 5, 221–228 (2018).
8. Tortora GJ, Funke BR, Case CL. Microbiology: an introduction. San Francisco, Calif. Pearson Benjamin Cummings; 2010, 10th ed.
9. ASHRAE. ASHRAE position document on airbone infectious diseases. 2014.
10. Barker J, Jones MV. The potential spread of infection caused by aerosol contamination of surfaces after flushing a domestic toilet. J Appl Microbiol. 2005;99(2):339–47. pmid:16033465
11. Holloman JL, Mauriello SM, Pimenta L, Arnold RR. Comparison of suction device with saliva ejector for aerosol and spatter reduction during ultrasonic scaling. J Am Dent Assoc. 2015;146(1):27–33.
12. Wax, R. S. & Christian, M. D. Practical recommendations for critical care and anesthesiology teams caring for novel coronavirus (2019-nCoV) patients. Canadian Journal of Anesthesia. 2020.
13. Rothe, C. et al. Transmission of 2019-nCoV infection from an asymptomatic contact in germany. N. Engl. J. Med. 2020.
14. Stadnytski, V., et al. The airborne lifetime of small speech droplets and their potential importance in SARS-CoV-2 transmission. PNAS. 2020.
15. Peng, X., Xu, X., Li, Y. et al. Transmission routes of 2019-nCoV and controls in dental practice. Int J Oral Sci 12, 9 (2020)
16. van Doremalen, N., et al. Aerosol and Surface Stability of SARS-CoV-2 as Compared with SARS-CoV-1. N Engl J Med 2020; 382:1564-1567.
17. Wei, J. & Li, Y. Airborne spread of infectious agents in the indoor environment. Am. J. Infect. Control 44, S102–S108 (2016).
18. Inside Dental Assisting. July/Aug 2014. 11(4). Centers for Disease Control and Prevention. Standards of Infection Control: Surfaces in the Dental Operatory Envi- ronment.
19. Upendran, A., & Geiger, Z. Dental Infection Control. 2020. Treasure Island (FL): StatPearls Publishing; 2020 Jan.
20. Whitehouse RL, Peters E, Lizotte J, Lilge C. Influence of biofilms on microbial contamination in dental unit water. J Dent. 1991;19:290–5.
21. Coleman DC, O'Donnell MJ, Shore AC, Russell RJ. Biofilm problems in dental unit water systems and its practical control. J Appl Microbiol. 2009;106:1424–37.
22. Shajahan, I., et al. Dental unit waterlines disinfection using hypochlorous acid-based disinfectant. J Conserv Dent. 2016 Jul-Aug; 19(4): 347–350.
23. Kim, Y., et al. Comparison of the preventive effects of slightly acidic HOCl mouthwash and CHX mouthwash for oral diseases. Biomedical Research 2018; 29 (8): 1718-1723.
24. Fast SM, González MC, Markuzon N. Cost-Effective Control of Infectious Disease Outbreaks Accounting for Societal Reaction. PLoS One. 2015; 10(8):e0136059.